Biomedical Materials
Current Projects:- Bioscaffolding for Neural Organoids
- Additive Manufacturing of Polymeric Microneedles for Stimuli-Responsive Biologic Delivery
- Additive Manufactured Implantable Car-T Factories for Localized Immunotherapy in Pediatric Brain Tumors
- Biomedical Microrobtics
- Additive Manufacturing of Minimally Invasive Drug-Eluting Microrods to Prevent Tumor Recurrence
Bioscaffolding for Neural Organoids
Personnel: Dr. Peter Serles (Postdoctoral Scholar)
90-Day old Human Cortical Organoids grown from Induced Pluripotent Stem Cells.
Neural organoids, clusters of brain tissue grown from human stem cells, represent one of the most promising tools for disease modelling, drug discovery, and understanding neurodevelopment as key models which replicate the neural characteristics of the human brain. These organoids remain rudimentary in their organization, long-term proliferation, and integration with other tissues due to a lack of vascularization and natural diffusion within the tissue, and homogeneous morphogen exposure during differentiation.
Bioscaffolding offers a promising integrated approach to improving these tissue outcomes through localized microfluidic delivery of nutrients and morphogens, material-level mechanical queues, and 3D tissue support. In this project, we developed a process to additive manufacture hydrogels to replicate the neural extracellular matrix and its associated nutrient delivery and concentrations. Growing the neural organoids in the scaffold creates an artificial environment which replicates the neural extracellular matrix with engineered life-support, enabling long-term growth and proliferation of human neural tissue for high fidelity drug screening, companion diagnostics, neurodevelopmental studies, and disease modelling.�
This project is in collaboration with the Quadrato Lab at USC.
Additive Manufacturing of Polymeric Microneedles for Stimuli-Responsive Biologic Delivery

Personnel: Analiese Wiedenbeck (Ph.D. student in Chemistry), and Jimmy (Minho) Kim (Ph.D. student in Chemical Engineering)
Microneedle patches offer a minimally invasive and highly effective method of drug delivery compared to subcutaneous injections and oral medications. Our work is focused on the use of advanced additive manufacturing techniques for the fabrication of microneedle patches with complex geometries to imbue unprecedented levels of control over drug delivery. We investigate the release kinetics and mechanical properties of these microneedles to ensure favorable release profiles and sufficient stiffness for skin penetration. We also conduct various in vitro and in vivo experiments to characterize biocompatibility and efficacy.
Additive Manufactured Implantable Car-T Factories for Localized Immunotherapy in Pediatric Brain Tumors
Personnel: Steven Chen (Ph.D. student in Medical Engineering)
Collaboration with Dr. Leo Wang (City of Hope).
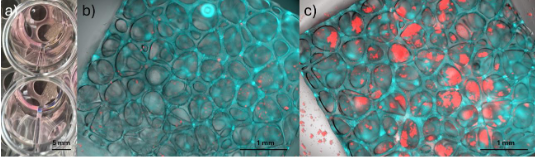
Pediatric brain tumors are difficult to treat with immunotherapy because they lack the baseline immune activity most treatments rely on. This project addresses that challenge through advanced 3D printing, which enables implantable materials that organize immune signals with high spatial precision. The aim is to fabricate small, implantable scaffolds that direct how immune cells encounter activation cues and antigens. 3D printing enables placement of distinct biological instructions at micrometer-scale resolution, as opposed to the uniform release profile of traditional materials. Such control reflects how antigen presentation naturally occurs, where signal layout, density, and timing shape immune cell development and activation. Presenting these cues in a defined sequence and location helps generate a more effective population of tumor-fighting cells at the disease site. The approach offers the potential for a focused and durable immune response with reduced systemic toxicity. In the long term, this spatially programmed strategy could extend to other cancers or immune-driven diseases that require controlled antigen presentation.
Biomedical Microrobotics
Personnel: Kaiyu Zhao (Ph.D. student in Medical Engineering)
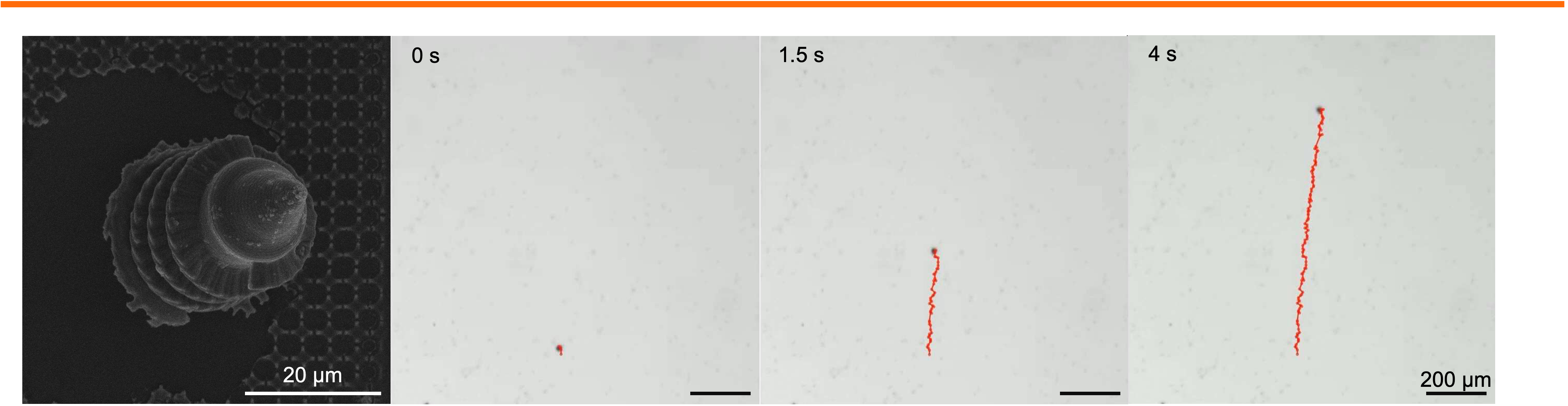
For biomedical microrobot, geometric design plays a central role in determining propulsion efficiency, maneuverability, and the ability of microrobots to traverse complex physiological environments. Acoustic and magnetic microswimmers, in particular, rely on precisely engineered three-dimensional architecture to effectively convert external fields into directed motion. Subtle variations in thickness, length, radius, aspect ratio, or surface anisotropy of the micro-robot can dramatically influence propulsion performance, highlighting the critical need for structural optimization in microrobot design. We employ two-photon lithography to fabricate microrobot with precisely tunable geometries, followed by additional processing to impart functionality. Using this approach, we seek to structurally optimize the microswimmers while systematically probing how the robotic construct influences the propulsion speed in viscous or heterogeneous biological media. Establishing these structure-function relationships will deepen our understanding of microscale locomotion and facilitate the insertion of uncovered physical principles into biomedical applications, such as accelerated drug delivery, enhanced tissue penetration, and improved navigation within constrained anatomical spaces.
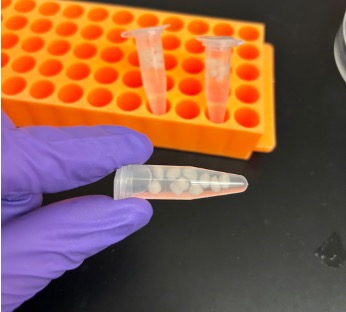
Additive Manufacturing of Minimally Invasive Drug-Eluting Microrods to Prevent Tumor Recurrence
Personnel: Caroline Dial (Ph.D. student in Medical Engineering)
Collaboration with Interventional Oncology Bioengineering Lab (City of Hope).
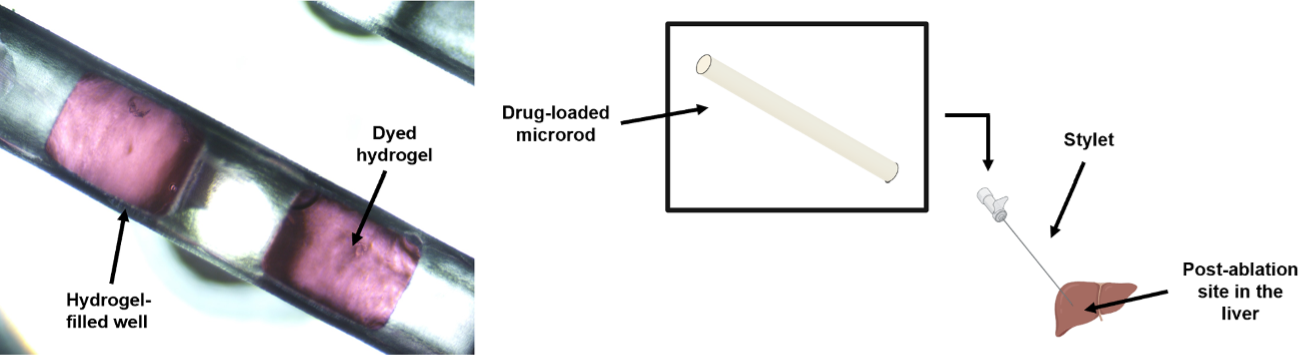
Ablation is a commonly used minimally invasive technique to treat liver tumors. Post-ablation tumor recurrence is a frequently occurring challenge because of the inevitable residual cancerous cells and the microenvironment-induced new tumor growth. Different approaches in preventing recurrence include adjuvant chemotherapy and immunotherapy, some of which are systemic and result in adverse side effects like severe gastrointestinal upset and flu-like symptoms. We are developing a microrod that can be delivered to a post-ablation site within the liver through a stylet in a minimally invasive procedure and can prevent side effects by providing localized delivery. We are utilizing additive manufacturing to fabricate a millimeter scale implant with micrometer scale features to enable precise site-specific drug placement and distribution. These microarchitectured implants recruit lymphocytes to the microrod, which would promote a natural anti-tumor immune response. Our microrods contain arrays of micro-wells filled with drug-loaded hydrogels, which regulate release. This is a new approach of utilizing additive manufacturing towards developing microarchitectured implants to precisely control drug distribution. This strategy would allow for a minimally invasive, targeted delivery system to create an anti-tumor response, currently a non-existent technology.